in Buying Guide
Reducing the Risk of Surgical Instruments
Each medical Device placed on the market, falls under a classification in the Medical Device Directive, which is determined by its function, use and period of use.
The main principle to understand is that the higher the Classification, more measures have to be taken to reduce this risk. As a general rule, all medical devices have to meet the essential requirements in the Directive. These must be fulfilled to allow a device to go on the market. As it is considered, if these are fulfilled, then the risk of selling the device has been substantially reduced.
For a class I instrument, the steps to reduce the risk of putting the device on the market are less and as a company, you can self-certify (register with the Dept. of Health). For a class II instrument, there are more requirements, which need to be assessed. A notified body (SGS in our case) must carry out this assessment (employed by us), to confirm the requirements are being met, as obviously if they weren’t, the consequence could be greater (i.e.death of the patient).
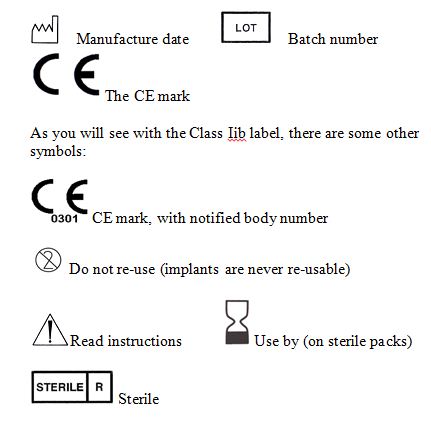
The main direct evidence you will see of this is product labelling and packaging. The Essential Requirements state that ‘Each device must be accompanied by the information to use it safely. This information must be set out on the packaging. I have included some examples of Instrument labelling. Typically, you will see these symbols, which must be present on class I surgical instruments.